Processes ensuring quality
We provide a framework for quality management at all stages of the patent granting process, thereby enabling continuous improvement.
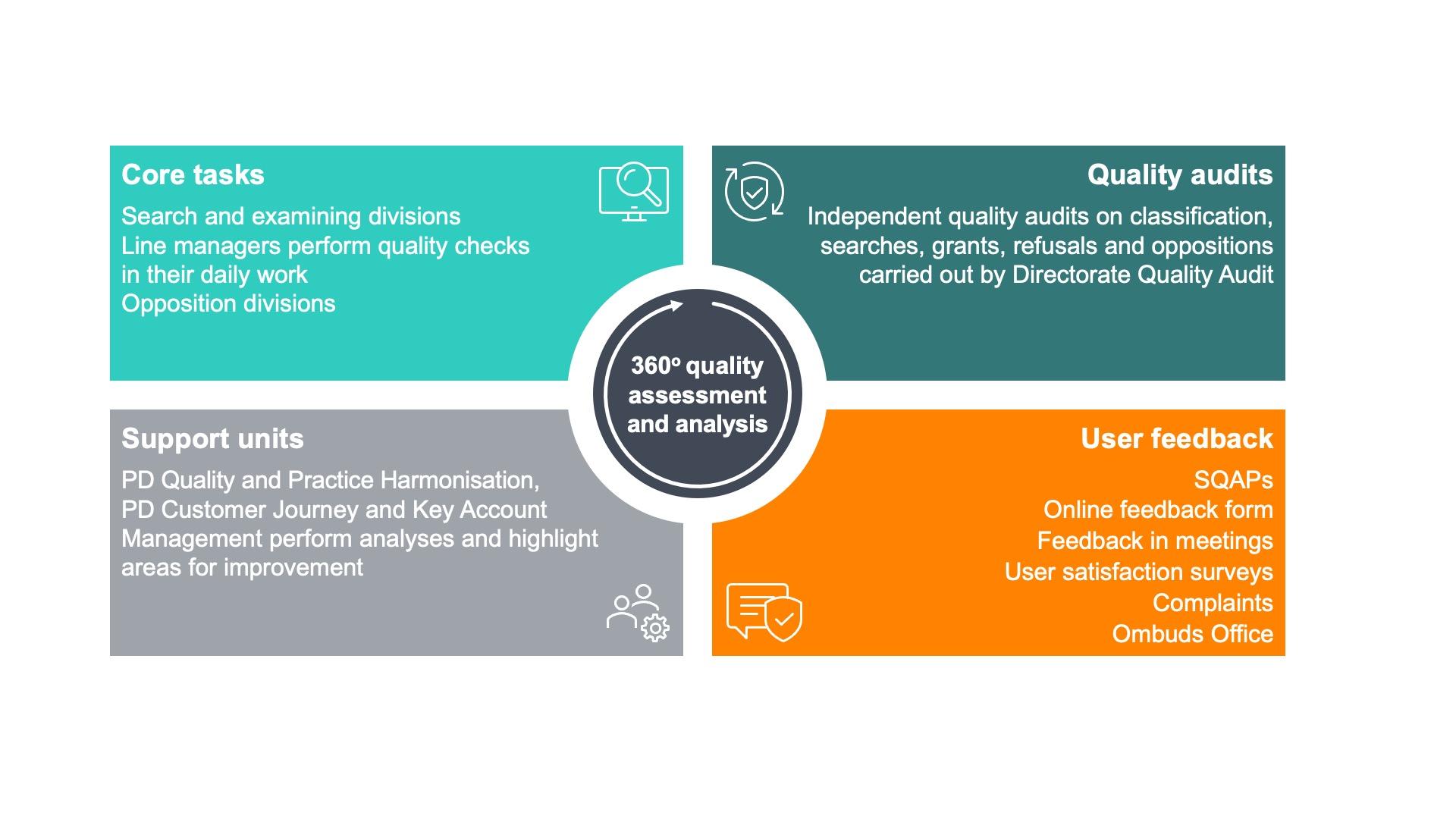
Internal quality-related data as well as user feedback are essential to capture feedback on our quality from many different perspectives and to identify areas where we can improve.
At the heart of EPO quality is the examining division. It ensures that all proposals for grants and all refusals are checked and signed by three technically qualified examiners, and it also conducts oral proceedings in examination. Similarly, opposition divisions comprise three examiners, at least two of whom were not members of the examining division that granted the opposed patent.
In addition to line manager checks carried out within our operational teams, the Directorate Quality Audit – which is independent of our operational teams – audits the quality of classification, search, examination and opposition work, as well as the work of formalities officers. This ensures regular and independent evaluation of how the divisions and formalities officers are applying the regulations. Our experienced team of experts audited 891 searches and 832 proposals for grants in 2022. The findings are used for continuous quality improvement of our products and services.
Quality is a joint responsibility, and we depend on our users to play their part by telling us where they think we can do better because we do act on the feedback we are given.
In addition, annual external audits on our quality management system are performed by an independent authority.
Findings give rise to various types of actions, which are a central part of our formal improvement cycle and are integrated in the annual Quality Action Plan.
As implemented by the EPO, our ISO 9001:2015 certified quality management system forms part of a comprehensive Integrated Management System (IMS) - giving you confidence in our processes and systems.
How we improve quality
Dedicated actions to continuously improve quality in all areas of the EPO arise from our Quality Action Plan. Quality actions are cascaded down and translated so that all staff receive set quality objectives that are relevant and actionable for them and their unit. Progress towards achieving the objectives is reviewed on a regular basis throughout the year.
Specific quality issues are monitored in a dedicated data base and managed as “Quality Initiatives”. This is a highly sophisticated approach involving the steps of:
- issue confirmation (is it really a systematic issue or a one-off error?)
- root cause analysis of the issue (what is causing the issue?)
- detailed action plan to eliminate the root cause
- effectiveness check on the actions taken (has the root cause been eliminated?)
- review by higher management on the issue, analysis, actions taken and effectiveness
For more information, please see the Quality Report 2022.