Q&A on patents and coronavirus
Questions covered:
- What are patents and what do they protect?
- Why do we need patents for pharmaceuticals?
- Does a patent grant a right to use the invention in practice?
- How long does patent protection last?
- How does the EPO decide which patents to grant?
- What public policy tools are available to ensure access to patented medicines?
- Can viruses such as the coronavirus be patented?
- How many patent applications have been filed at the EPO for technologies related to coronaviruses?
1. What are patents and what do they protect?
Patents provide legal protection for technical inventions in all fields of technology. They grant their owners an exclusive right to an invention in one or several countries for a limited period of time, usually up to 20 years from the date of filing of the application. Patents need to be maintained through the payment of annual renewal fees to the national patent offices of the countries in which they are valid. Once a patent is no longer maintained or its term has expired, the invention falls in the public domain and can be used by anyone.
To obtain patent protection in the first place, an inventor must fully disclose his invention in the application. Patent applications are published 18 months after the priority date, and because they are available publicly, create transparency on the latest technological developments. This allows researchers in the same field to build on the inventions of others. The patent system therefore plays an important role in stimulating innovation, including in medical technology or pharmaceuticals, where a lot of patent applications are filed every year.
At the European Patent Office, after publication of the patent application, communications between a patent applicant and the office are made available to the public through the online European Patent Register. So anyone can monitor developments in every case pending before the EPO. Moreover, the EPO's free online patent database Espacenet, provides access to over 130 million documents, making it one of the largest single sources of information about inventions and technical developments from all over the world.
2. Why do we need patents for pharmaceuticals?
Patents provide an important incentive for the research and development of new medicines by enabling inventors to attract investment and recoup their R&D expenses. This is especially important in areas where product development costs are high or substantial start-up investment is required. Patents give their owners the right to prevent third parties, e.g. competitors, from commercially using their invention without their consent. But it is still possible for third parties to use or sell an invention with the help of a licensing agreement. Patents are therefore very often used to build bridges through co-operation among inventors, to pool R&D activities of businesses and to create standards in certain technologies, for instance. Patents are also an effective barrier to the illicit copying of medicines and the health risks associated with counterfeit versions.
3. Does a patent grant a right to use the invention?
Patents do not give their owners permission to make use of their invention in any form. The use and exploitation of technology remain subject to national laws and regulations. In the field of pharmaceuticals, other bodies are responsible for decisions on the use of medicines (European Medicines Agency, US Food and Drug Administration, etc.).
4. How long does patent protection last?
According to the European Patent Convention, a European patent expires at the latest 20 years from the date of filing. In the European Union and some European countries it is possible to extend the protection for medicines to compensate for the long market approval processes at the national or European medicines agencies. Once a patent has expired, the invention falls in the public domain and can be used by anyone without paying royalties: According to information published by the World Intellectual Property Organization (WIPO), 95% of the medicines on the World Health Organization's List of Essential Medicines are no longer protected by a patent and are in the public domain (source: WIPO study, 2016).
5. How does the EPO decide which patents to grant?
At the EPO, every patent application is subject to a rigorous search and examination procedure to decide whether a patent can be granted. The relevant patent law for European patents is the European Patent Convention (EPC), which stipulates that patents can be granted only for technical inventions that are new, involve an inventive step and are industrially applicable. The member states of the EPO have largely aligned their national laws with the legal standards of the EPC.
The grant procedure is carried out by three highly-qualified scientists or engineers working as patent examiners specialised in the relevant technology. Once applicants receive from the EPO a preliminary opinion about their chances of obtaining a patent for their invention, they can take an informed decision on whether they want to pursue their application further. Consequently, a large number of the patent applications filed do not lead to the granting of a patent. Furthermore, the scope of protection of many of the patents that are granted will have been narrowed down during the examination process in order to meet the EPC criteria.
6. What public policy tools are available to ensure access to patented medicines?
The patent laws of many countries include safeguards to prevent patent holders from abusing their position in the market. Thus anti-competitive practices are prohibited and compulsory licencing can be imposed by public authorities. Compulsory licensing of patents allows governments to override patent holders' rights to exclude all others from using their inventions when there is significant public interest, for example in relation to providing life-saving medicines. It has not been used frequently in Europe (for a general overview, see also the EPO's publication Compulsory Licensing in Europe: a country-by-country overview, 2018). The grounds for granting compulsory licences are limited, and their use is subject to significant judicial or administrative scrutiny in each of the countries considering such measures.
7. Can viruses such as the coronavirus be patented?
Scientists and researchers in the medical field looking to develop a new diagnostic test, treatment or vaccine may isolate biological material such as a virus, and seek patent protection for it and its use in the prevention, diagnosis or cure of a disease. European patent law does allow patent protection for biological material such as viruses and bacteria, provided the biological material is isolated from its natural environment or produced by means of a technical process. In order to be patentable, the isolated virus (or its genome or parts thereof) should, in addition to being new (i.e. not recognised as having existed before), provide an inventive solution to a technical problem, such as the development of a diagnostic kit to detect an infection or the production of a vaccine to protect against the infection. Such patents describe the invention and often the virus itself in detail, thus disclosing the patented technology and ensuring that the diagnostic kit or the vaccine can be reproduced by other specialists and become the basis for further developments. It should also be noted that European patent law takes ethical considerations into account, preventing the patenting of inventions whose commercial exploitation would be contrary to "ordre public" or morality.
8. How many patent applications have been filed at the European Patent Office for technologies related to coronaviruses?
While we do not yet have figures for patent applications related to the current coronavirus outbreak, a search in our patent databases (see Espacenet) shows that from 1978 to 2016 (the year for which the most complete data is available) the European Patent Office received a total of 390 patent applications for technologies related to coronaviruses (see Graph below). This includes patent applications for various aspects of inventions related to coronavirus (e.g. diagnostics, detection, vaccines, pharmaceuticals for the treatment of infections) as well as for all coronavirus types, including those that infect animals and humans. During the same time period, the European Patent Office granted 152 European patents related to such inventions.
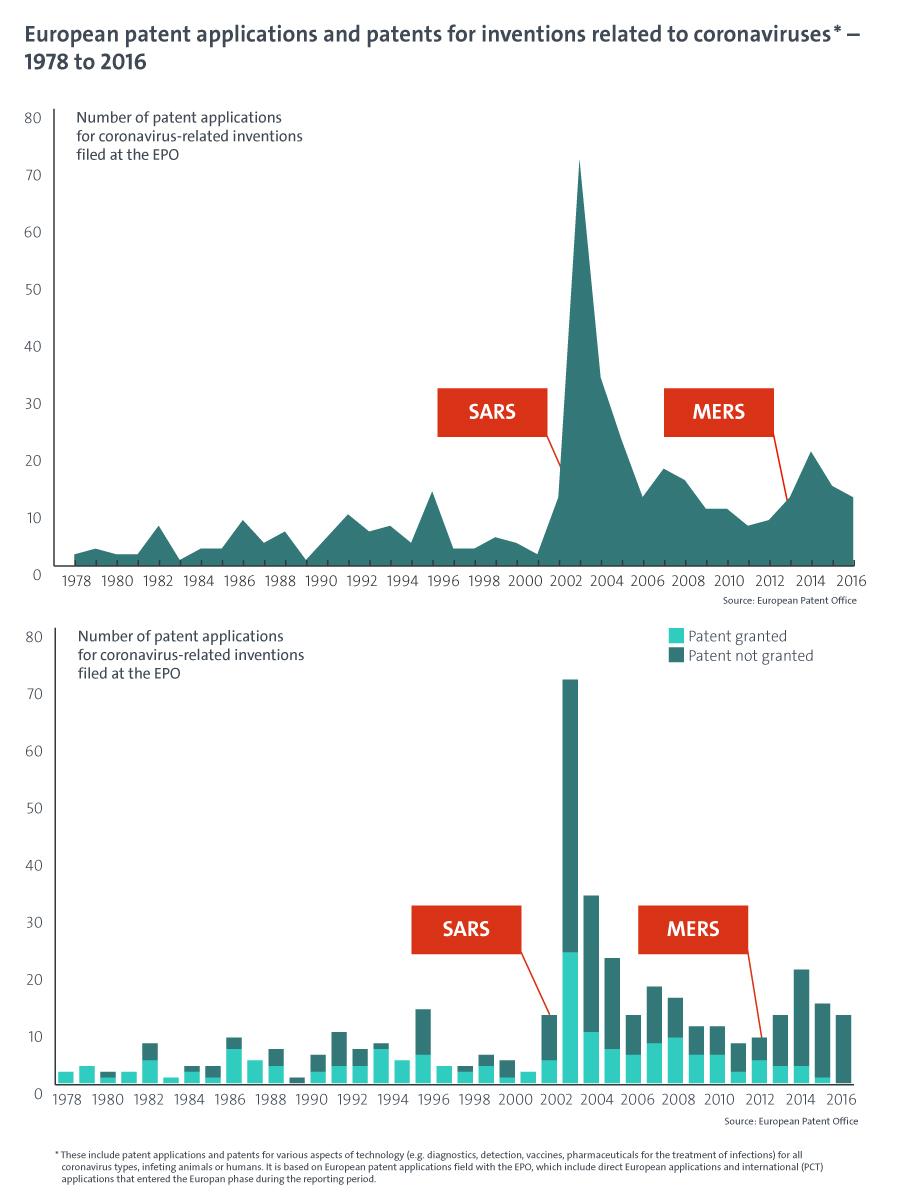
The statistics show that the patenting of technologies associated with coronaviruses is increasing overall. Various outbreaks of new coronavirus infections have led to a sharp increase in patenting activity in the field, with peaks in patent filings visible for the years immediately following the severe outbreaks of SARS (2002) and, to a lesser extent, MERS (2012). Since 2014, demand for patents in the field has remained relatively high. The current outbreak of the coronavirus disease caused by SARS-CoV-2 is expected to spur a rapid and strong increase in patenting activity as researchers and companies work on developing vaccines, diagnostic tests and medicines to tackle the pandemic.
For more information, please contact the EPO press team: press@epo.org