Helen Lee
Diagnostic kits for developing countries
Winner of the European Inventor Award 2016 in the Popular Prize category
Lee have unlocked an unprecedented level of infectious disease management in sub-Saharan Africa and other developing regions. Administered as a simple blood plasma test, the invention delivers results within a matter of minutes - requiring no trained personnel or clinical infrastructure - while also monitoring levels of viral load in the blood as an important feedback signal during medical treatment.
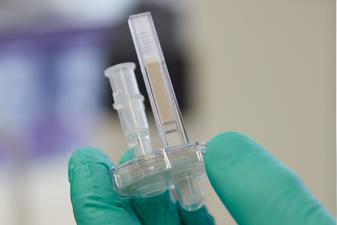
Lee achieved her breakthrough by focusing on a testing method that would yield results visible to the naked eye, instead of relying on costly microscopes or visualisation techniques. Her simple test consists of mixing a patient's blood with so-called nucleic acid-based assays, a combination of chemicals in a disposable cartridge that will change colour in the presence of viral RNA in blood plasma samples. Unlike other tests, the cartridges do not require cold storage or transport - making them perfectly suited for conditions in Africa - and the kits can be stored at temperatures of up to 37°C for nine months.
Societal benefit
While the spread of HIV is largely contained in developed countries, the disease continues to proliferate in regions such as sub-Saharan Africa. Here, around 25 million people are currently living with the virus, accounting for 70% of the global total according to the World Health Organisation. Diagnosis and treatment are keys to stopping the pandemic, but a lack of clinical infrastructure continues to impede progress.
Inexpensive and precise, Lee's invention solves three fundamental problems of infectious disease outreach in resource-poor areas. Firstly, the tests deliver results without need for refrigeration, trained personnel or elaborate equipment - perfect for "test-and-treat" facilities in the developing world. Secondly, "instant" test results prevent patients being "lost to care" - in other words leaving before diagnosis results are ready - which can account for 30-70% of all patients. Thirdly, the tests monitor viral load in patients' blood, critical for gauging drug dosages for treatment.
Economic benefit
Marketed by Lee's start-up company Diagnostics for the Real World Ltd (DRW) as the SAMBA (Simple AMplification Based Assay) system, the tests have been used to screen 40 000 people for HIV in Malawi, Uganda and a growing number of other sites in co-operation with Doctors Without Borders, among others. A SAMBA machine can run up to four samples at a time, costing EUR 15 (USD 17) for each test. It runs on electricity but can switch to eight-hour batteries during outages. The SAMBA HIV Assay for therapy monitoring has recently received the CE regulatory approval for use in the European territories.
Headquartered in Sunnyvale, California, and in Cambridge, UK, as a non-profit academic unit at the University of Cambridge, Diagnostics for the Real World has successfully raised around EUR 60 million (USD 65 million) in research and health grants to date from organisations such as UNITAD, NIH and the Wellcome Trust. DRW operates under a 15% cap on profits to develop point-of-care diagnostic assays for resource-strained regions. Point-of-care diagnostics are an international growth market, estimated at EUR 12.7 billion (USD 14.1 billion) in 2013 and expected to grow at a CAGR of 9.7% to EUR 28.7 billion (USD 32.7 billion) by 2022.
How it works
The SAMBA (Simple AMplification Based Assay) test is conducted by drawing a blood sample from a patient and then feeding it into a machine that inserts a dipstick - similar to a pregnancy test - coated with chemicals known as nucleic acids. These chemicals do not require refrigeration and "amplify" the viral RNA in a blood sample to the point of generating a visible colour change in specific patterns on the dipstick: two red lines on the stick indicate a positive sample, one line a negative, and no line an invalid test.
By detecting the virus directly, and not antibodies in the blood, SAMBA also unlocks HIV testing for infants under the age of 18 months. This is a major benefit, as these infants have not yet developed antibodies to fight the disease and will likely die before their second birthday without treatment. The SAMBA platform is currently expanded to detect influenza A and B as well as a combination test for chlamydia and gonorrhoea. SAMBA II, which offers faster and even more flexible testing, is in clinical trials.
The inventor
Helen Lee was born in China and through her marriage holds a French passport. Her career in diagnostics began at the Centre National de Transfusion Sanguine (French National Blood Transfusion Centre) in Paris. After a successful stint at Abbott Laboratories managing over 100 people and an annual budget of roughly EUR 17 million (USD 20 million) as general manager of the Probe Diagnostics Business Unit, Lee withdrew from the enterprise world in the mid-1990s to focus on research.
In 1996, she started her ongoing pursuit of technologies and diagnostic assays for resource-strapped regions as the head of the Diagnostics Development Unit (DDU) at the University of Cambridge. The unit has filed 12 families of patent applications, with around 20 granted national patents.
Her work has been recognised with the Lord Lloyd of Kilgerran Award (2005), European Women of Achievement Award (2006), British Female Inventor in Industry Award (2006) and the 2007 Asian Women of Achievement Award. Lee is an avid football fan - supporting the Arsenal "Gunners" - and enjoys cooking and Chinese painting.
Did you know?
Although diagnostic tools can have paradigm-changing effects in fighting pandemics such as HIV, their market potential is rather limited compared to therapeutic drugs. The global market for rapid medical diagnostic kits - including Lee's inventions - was estimated at EUR 18.5 billion (USD 20.4 billion) in 2015 (ResearchandMarkets). That same year, one single company on the pharmaceutical drugs market - market leader Pfizer - reported sales of EUR 43.1 billion (USD 47.4 billion).
In that light, Helen Lee could have pursued a more lucrative career on the global pharmaceuticals market, which posts sales to the tune of EUR 959.43 billion (USD 1 057.2 billion) each year (Statista). Instead, the researcher continues her focus on helping patients in poor regions: "If all we do is develop prototype assays or publish papers, then we will have failed," she said in an interview.
Inventors revisited
In 2020, the EPO reconnected with former finalists and winners for their views on trends in innovation and intellectual property, and a rare glimpse at cutting-edge new research and inventions.

A test to change the world
Helen Lee set herself a seemingly impossible task: develop an easy‑to‑use medical diagnostic device that operates in extreme conditions and gives accurate results in rapid time. Helen's SAMBA II has been deployed in several developing regions and is part of programmes to detect mother‑to‑child transmission of HIV.

Talk innovation
Helen Lee left a career in industry to work on an invention that would be a game-changer in treating viruses such as HIV. Her device is now deployed in several developing regions and is part of programmes to detect mother-to-child transmission of HIV. In 2020, Helen and her team developed a test for SARS-CoV-2, giving medical professionals an important weapon in fighting the spread of COVID-19.
Media gallery
Contact
European Inventor Award and Young Inventors Prize queries:
european-inventor@epo.org Subscribe to the European Inventor Award newsletterMedia-related queries:
Contact our Press team#InventorAward #YoungInventors